Fly maggot disease / Myiasis
Myiasis or fly maggot disease describes the infestation of live animals with fly maggots. The disease is most commonly caused by flies from the families Calliphoridae, Sarcophagidae, Chloropidae and Muscidae. As a rule, open wounds but also body openings are affected. Dead tissue and dirt from feces or urine particularly attract flies. The embedded maggots do not initially penetrate too deeply as they prefer the oxygen-rich environment. The deeper the hole, the deeper the maggots penetrate. Myiasis in humans and livestock, especially sheep, is more common in the tropics than in temperate zones. A general distinction is made between obligate parasites and facultative parasites. The obligate parasites grow in nature only on healthy tissue of living hosts, while the facultative parasites usually occur in carrion, feces or decaying plant material and are also saprophages.
Calliphoridae: Lucilia bufonivora (toadfly)
Lucilia is a genus of blowflies (Calliphoridae) consisting primarily of saprophagous and facultative parasites. The only obligate parasite of amphibians among the blowflies is Lucilia bufonivora (toadfly). This type of fly is not new to science, but was described on toad carcasses over 100 years ago. The distribution area includes Europe, North America, Asia and also North Africa. The toadfly has been detected most frequently in Central Europe, particularly the Netherlands and parts of Germany (North Rhine-Westphalia). However, there is too little evidence, so much about its distribution and frequency is unknown. By far the most affected species in Europe are the common toads, especially the larger female toads. There are rarer reports of natterjack toads, green toads, common frogs, water frog complexes and midwife toads. The toadfly has been found very rarely on the tree frog and the fire salamander.
Behavior
In the summer months, the female toadfly lays up to over 100 eggs on the backs of live amphibians (compare Figure 1).

© S. Swoboda
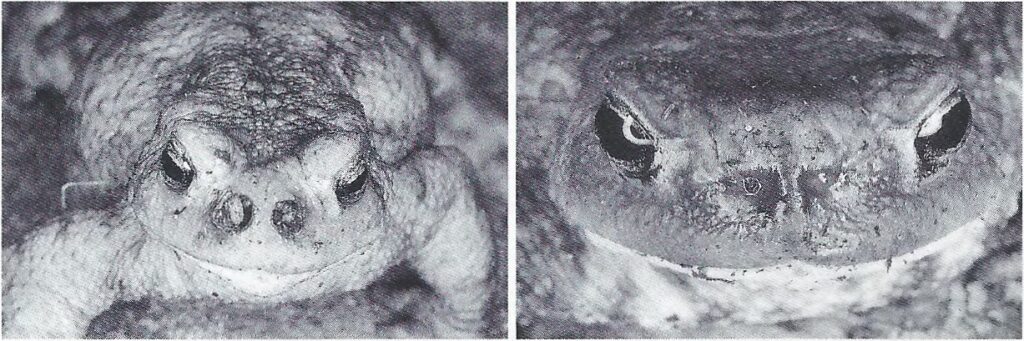
The maggots, which hatch after a few hours depending on the temperature, crawl to the nostrils and penetrate the head. They initially lead to the expansion and destruction of the nostrils (compare Figure 2).

© R. Stawikowski
Im späteren Verlauf werden andere nahegelegene Organe zerstört, bis die Amphibie schließlich stirbt. Während die Krötengoldfliegen tagaktiv sind, sind die Erdkröten nachtaktiv. Es ist unbekannt wie die Krötengoldfliegen ihre Wirtstiere lokalisieren. Wissenschaftler vermuten eine geruchliche Ortung der Amphibien. Befallene Erdkröten sind auch im Frühstadium meist an asymmetrischen, leicht nässenden Nasenöffnungen und untypischem, tagaktiven Verhalten erkennbar. Eine Übersicht zum Krankheitsverlauf ist in Abbildung 3 gezeigt.

© B. Thiesmeier & K. Weddeling
[Download as .xcf file] [Download as .psd file] [Download Original as .jpg file]
Later, other nearby organs are destroyed until the amphibian finally dies. While the toadflies are active during the day, the common toads are active at night. It is unknown how toadflies locate their hosts. Scientists suspect that amphibians can be located by smell. Even in the early stages, infected common toads can usually be recognized by asymmetrical, slightly weeping nostrils and atypical, diurnal behavior. An overview of the disease progression is shown in Figure 3.
Healing success
Der Befall endet für den Wirt normalerweise tödlich. Die Eier haften stark an der Amphibienhaut und gehen kaum verloren. Befreien kann sich die Kröte nur mit einer Häutung. Jüngere und schneller wachsende Kröten häuten sich öfters als ältere Exemplare. Da die Eier rasch Schlüpfen ist eine Befreiung durch Häutung selten oder eher unwahrscheinlich. Die Eier mit Pinsel zu entfernen misslangen, die Entfernung per Zahnbürste ist sehr mühsam und mit großem Aufwand verbunden. Die Entfernung mittels spitzer Pinzette ist allerdings möglich (Janzen 1994 [1]). Die Fliegeneier haften nicht an allen Amphibienarten so stark wie hier beschrieben. Bei einem Laubfrosch (Hyla arborea) waren die Eier leicht abstreifbar (Meisterhans et al. 1970 [2]). Sind die Maden bereits in die Nasenlöcher eingedrungen kann der Kröte nur noch geholfen werden, in dem alle Maden mit einer spitzen Pinzette entfernt werden. Dr. P. Janzen hatte einer Erdkröte 35 Maden aus Nase und Gaumenbereich entfernt. Nach 34 Tagen waren die Nasenlöcher zumindest äußerlich wieder verheilt (vgl. Abbildung 4).
The infestation is usually fatal for the host. The eggs adhere strongly to the amphibian skin and are rarely lost. The toad can only free itself by molting. Younger and faster-growing toads shed their skin more often than older specimens. Since the eggs hatch quickly, release through molting is rare or unlikely. It was not possible to remove the eggs with a brush; removing them with a toothbrush is very annoying and involves a lot of effort. However, removal using sharp tweezers is possible (Janzen 1994 [1]). The fly eggs do not adhere to all amphibian species as strongly as described here. In a tree frog (Hyla arborea), the eggs were easy to remove (Meisterhans et al. 1970 [2]). If the maggots have already penetrated the nostrils, the only way to help the toad is to remove all the maggots with sharp tweezers. Dr. P. Janzen had removed 35 maggots from the nose and palate of a common toad. After 34 days, the nostrils had healed again, at least externally (compare Figure 4).
© P. Janzen
The maggots don’t like being under water and can be lured out with the help of the water. It can be assumed that some maggots follow the oxygen gradient under water and leave the toad on their own (compare Figure 5). At least in North America it was observed that frogs bathing in water were able to free themselves from the maggots (Roberts 1998 [3]). However, the maggots probably did not colonize the nostrils there and could therefore be kept under water by the frog for longer.
Release incurably ill toads of their pain.
If the infestation has already progressed too far, the animal should be relieved of the pain. Keeping a toad alive in the wild without a sense of smell is also questionable. For the painless euthanasia of the amphibians to be released, the use of the prescription substance MS-222 and not the “Cooling then Freezing” method (Shine et al. 2015 [4]) is recommended (Ms. dipl. med. vet. Petra Lohmann, personal communication August 2, 2021).

© Wikipedia User Jona23d
Another variant of Lucilia bufonivora with a different myisasis pattern
In North America but also in Eurasia, another variant of L. bufonivora was originally permanently referred to as L. silvarum (forest toadfly). L. bufonivora and L. silvarum are very similar in terms of phenotype and distribution and can only be distinguished genetically based on correctly selected DNA fragments or complete sequencing. Unfavorable DNA fragments were compared and the second variant of L. bufonivora was confused with L. silvarum for a long time. The second variant of L. bufonivora is mainly saprophagous, but is also rarely known as a facultative parasite of amphibians. The eggs are laid on the back of the amphibian as with the traditional L. bufonivora. The hatched maggots of the second variant do not migrate to the nostrils but instead try to penetrate directly through the skin. Some of the larvae migrate further under the skin to gather in a single place. Maggots that hatch later crawl to the already necrotic tissue and penetrate there. The necrotic tissue attracts more flies and also total different fly species due to the smell.
L. silvarum is therefore no longer considered a facultative parasite of amphibians, at least in the area studied in the northern hemisphere, but is considered to be strictly saprophagous. However, it has not yet been investigated whether the second variant of L. bufonivora from North America is also present in Eurasia, or whether it is a third variant of L. bufonivora there (Whitworth et al. 2020 [5] & Whitworth, persönliche Kommunikation 09. August 2021).
Other facultative parasites of the genus Lucilia
Lucilia elongata has been incorrectly confirmed three times as a facultative parasite of amphibians, but incorrect genes were compared in these detections. Since L. elongata is genetically and morphologically very similar to L. bufonivora, this species is still suspected to be a facultative parasite of amphibians in North America (Whitworth, personal communication August 9, 2021).
Lucilia thatuna is interesting because of its distribution in the swamp areas. However, there is currently no direct detection of L. thatuna on amphibians.
Lucilia ampullacea was detected on a common toad in Munich (Glaw et al. 2014 [6]). In L. ampullacea, as with all facultative parasites, the maggots penetrate through the skin and not via the nostrils.
Detection made difficult by secondary infestation
A toad infected with the common L. bufonivora is often secondarily attacked by facultative parasites later in the course of the disease. The fly maggots generally prefer to pupate in the soil and not in the amphibians themselves. Therefore, when taking a sample, the ground below the dead amphibian should always be checked for maggot pupae.
Myiasis in South and Central America
Sarcophagidae: Lepidodexia sp.
In the Neotropical region (South and Central America), flesh flies (Sarcophagidae) of the genus Lepidodexia are particularly responsible for myiasis in amphibians. Most detections derive from Lepidodexia bufonivora. Other Lepidodexia species that have been identified in amphibians are Lepidodexia centenaria and the newly described species Lepidodexia adelina. Sometimes only the genus Lepidodexia or even just the family Sarcophagidae was identified. There are also over a hundred other species of the genus Lepidodexia. The very warm, humid climate, and the associated rapid growth of the maggots and accelerated decomposition, as well as the often very small size of the amphibians, make it very difficult to detect further Lepidodexia sp.
The video below (compare Video 1) shows a Lepidodexia sp. attacking a tree frog (Hylidae) of the species Bokermannohyla hylax that is resting and exposed during the day. It is most likely the only video that shows a fly laying eggs or maggots on a live amphibian. The fate of the frog is unknown, neither maggot nor frog was collected, so there is only one record of the genus Lepidodexia. In Bokermannohyla caramaschii and Bokermannohyla luctuosa there is also only evidence of Lepidodexia sp. Maggots were collected and unfortunately killed immediately in 70% ethanol. Killing the maggots immediately is a mistake; the fully developed flies, in contrast to the maggots, are much more easily distinguishable by the entomologist using a microscope or high-resolution camera. In another case, maggots in Pristimantis thectopternuse were also killed immediately in 70% ethanol. There the entomologists were only able to identify the family Sarcophagidae. In the worst case, it cannot even be assigned to a family. Without any clues, detection may even require complete DNA sequencing, which is too expensive. After a rough classification by an entomologist, you can work with specific primers. There is no need to sequence anything, just determine whether the primer is bound to the DNA, as with the PCR Corona test.
© Rafael Guadeluppe
- Detection of unidentified fly:
- Detection of Sarcophagidae:
- Centrolenidae: Hyalinobatrachium fleischmanni (Medina et al. 2009 [8])
- Hylidae: Boana atlantica (Oliveira et al.2012 [9]), Scinax fuscovarius and Scinax ruber (Souza-Pinto et al. 2015 [10]), Dryaderces inframaculata (Pinto et al. 2017 [11]), Dendropsophus schubarti in Peru (Junes et al. 2019 [12])
- Strabomantidae: Pristimantis thectopternus (Gómez-Hoyos et al. 2012 [13])
- Bufonidae: Rhinella diptycha (Souza-Pinto et al. 2015 [10]), Rhinella alata (Kelehear et al. 2020 [14])
- Leptodactylidae: Leptodactylus latrans (Müller et al. 2015 [15]).
- Detection of Lepidodexia sp.
- Hylidae: Bokermannohyla hylax, Bokermannohyla caramaschii und Bokermannohyla luctuosa (Lemos et al 2019 [16])
- Detection of Lepidodexia bufonivora
- Detection of Lepidodexia centenaria
- Hylidae: Hypsiboas beckeri in Brasil (Mello-Patiu et al. 2010 [20]). L. centenaria is apparently host-specific to H. beckeri and native to high-altitude regions of the Brazilian Atlantic rainforest, in contrast to the habitat and host generalist Lepidodexia bufonivora (Mello-Patiu et al. 2010 [20]).
- Detection of Lepidodexia adelina
- Leptodactylidae: Adenomera diptyx, Leptodactylus elenae and Physalaemus albonotatus are all endemic to Argentina and have described a new Lepidodexia species (Mulieri et al. 2018 [21]).
Sarcophagidae: Peckia (Sarcodexia) lambens
Peckia (Sarcodexia) lambens also belongs to the family Sarcophagidae. P. lambens is widespread and is one of the most important forensic flesh flies for determining the time spent on corpses in South and Central America. P. lambens is a parasite of the butterfly caterpillar of Spodoptera frugiperda (fall armyworm), a pest of maize monocultures. S. frugiperda has been introduced into numerous countries in Africa and into India and China, and it is imaginable that P. lambens could also enter other areas unintentionally or intentionally.
- Detecion of Peckia (Sarcodexia) lambens
- Dendrobatidae: Ameerega cainarachi and Ameerega trivitatta in the San Martín region of Peru (Hagman et al. 2005 [22])
Phoridae: Megaselia scalaris
Neben der Familie der Fleischfliegen ist auch die Art Megaselia scalaris, der Familie der Buckelfliegen (Phoridae), für Myiasis bei Amphibien in Südamerika bekannt. M. scalaris wird bis 2-3 mm lang und kann sich durch den Boden bis zum Sarg graben, was ihr den Spitznamen “coffin fly” zu Deutsch “Sargfliege” gab. M. scalaris ist in der Forensik sehr wichtig und oftmals sogar das einzige forensische Beweismittel, das zur Verfügung steht, wenn die Leiche verdeckt oder an einer Stelle versteckt ist, die für andere Insekten schwer zu erreichen ist. Desweiteren wird M. scalaris häufig in der Forschung und im Labor verwendet, da sie leicht zu kultivieren ist. Die Nachweise (siehe unten) von M. scalaris bei Amphibien sind bisher selten. Von Boana faber wurden allerdings gleich drei betroffene Exemplare gefunden, zwei davon sind unten abgebildet (vlg. Abbildung 6).
In addition to the flesh fly family, the species Megaselia scalaris, of the humpback fly family (Phoridae), is also known for myiasis in amphibians in South America. M. scalaris is up to 2-3 mm long and can burrow through the ground to the coffin, which gave it the nickname “coffin fly”. M. scalaris is very important in forensic science and is often the only forensic evidence available when the body is obscured or hidden in a location difficult for other insects to reach. Furthermore, M. scalaris is often used in research and laboratories because it is easy to culture. The evidence (compare below) of M. scalaris in amphibians is so far rare. However, three affected specimens of Boana faber were found, two of which are shown below (compare Figure 6).
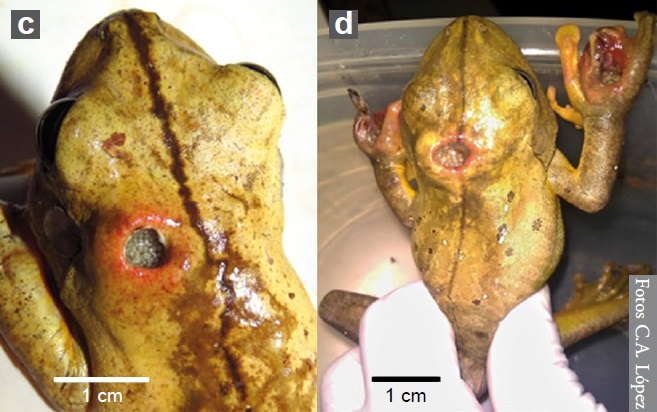
© C. A. López
- Detection of Phoridae: Megaselia scalaris
Myiasis in Australia
In Australia, at least 10 species of the genus Batrachomyia (Australian frogflies) of the stem fly family (Chloropidae) are known to infest frogs of the families Hylidae and Myobatrachidae. The eggs of Batrachomyia sp. require high humidity and are placed close to the frogs. When a maggot reaches a frog, it attempts to nest there and grows to a length of up to 10 mm in the frog. Since a frog is usually only attacked by one or a few maggots (≤ 4), up to 90% of the frogs survive (Mullen et al. 2009 [25]). There is the most evidence for the genus Litoria, the most species-rich genus of Australian tree frogs. An infected specimen of Litoria genimaculata is shown in Figure 7 below.

© Jean-Marc Hero
An Australian frog fly was also discovered in Papua New Guinea, an island nation next to Australia. The species is called Batrachomyia krausi and does not occur in Australia itself or has not yet been detected there. B. krausi has been identified as a parasite of the frog Papurana supragrisea of the family Ranidae. It is the first evidence of infestation of frogs of the family Ranidae by Batrachomyia (Evenhuis 2006 [26]).
Conclusion
Die Myiasis in Amphibien ist zwar ein ekliges Phänomen, aber Bedrohungen für die Existenz gewisser Amphibienarten wurden bisher noch keine nachgewiesen. Generell für Mensch und Tier ist wichtig Wunden richtig zu behandeln und eine gewisse Hygiene zu beachten, insbesondere in den tropischen Gebieten. In seltenen Fällen können auch Stubenfliegen (Musca domestica) zum Problem werden. Zum Bsp. bei einer Persischen Trughornviper wurde eine durch Musca domestica verursachte Wundmyiasis festgestellt (Dehghani et al. 2012 [27]).
Although myiasis in amphibians is a disgusting phenomenon, no threats to the existence of certain amphibian species have yet been demonstrated. In general, it is important for people and animals to treat wounds correctly and to observe a certain level of hygiene, especially in tropical areas. In rare cases, house flies (Musca domestica) can also become a problem. For example, wound myiasis caused by Musca domestica was found in a Persian horned viper (Dehghani et al. 2012 [27]).
Flies that cause myiasis also harm our livestock, especially sheep, which are very susceptible. As is well known, sheep meat is again and again riddled with maggots. Eradication of flies specialized in myiasis should therefore be considered.
List of sources
[1] P. Janzen, 1994: Heilungserfolg bei Erdkröten (Bufo bufo) mit Lucilia-Befall (Diptera: Calliphoridae). Salamandra Vol 30: 265–267 link
[2] Meisterhans, K. & H. Heusser, 1970: Lucilia-Befall an vier Anuren-Arten. Mitteilungen der Schweizerischen Entomologischen Gesellschaft 43(1): 41-44
[3] W. R. Ropberts, 1998: The calliphorid fly (Bufolucilia sylvarum) parasitic on frogs in Alberta. Alberta Naturalist 28: 48.
[4] R. Shine, J. Amiel, A. J. Munn, M. Stewart, A. L. Vyssotski, J. A. Lesku, 2015: Is “cooling then freezing” a humane way to kill amphibians and reptiles? Biol Open 15 July 2015; 4 (7): 760–763 link
[5] T. L. Whitworth, M. G. Bolek, G. Arias-Robledo, 2021: Lucilia bufonivora, Not Lucilia silvarum (Diptera: Calliphoridae), Causes Myiasis in Anurans in North America With Notes About Lucilia elongata and Lucilia thatuna. Journal of Medical Entomology, Volume 58, Issue 1, January 2021, Pages 88–92 link
[6] F. Glaw, J. Moriniere, D. Doczkal, 2014: Myiasis of the common toad (Bufo bufo) caused by the blowfly Lucilia ampullacea. Zeitschrift fur Feldherpetologie 21(1):83-95 link
[7] R. von May, E. Biggi, H. Cárdenas, M. I. Diaz, C. Alarcón, V. Herrera, R. Santa-Cruz, F. Tomasinelli, E. P. Westeen, C. M. Sánchez-Paredes, J. G. Larson, P. O. Title, M. R. Grundler, M. C. Grundler, A. R. Davis Rabosky, D. L. Rabosky, 2019: Ecological interactions between arthropods and small vertebrates in a lowland Amazon rainforest. Amphibian & Reptile Conservation 13:65–77 link
[8] D. Medina, M. Rivera, R. Cossio, E. Medina, S. Bermúdez, 2009: Primer registro de miasis por Sarcophagidae (Diptera: Oestroidea) en Hyalinobatrachium fleischmanni (Anura: Centrolenidae) de Panamá. Revista Mexicana de Biodiversidad 80:263–264 link
[9] R. M. de Oliveira, C. V. de Mira Mendes, D. S. Ruas, M. Solé, L. C. Pinho, R. Rebouças, 2012: Myiasis on Hypsiboas atlanticus (Caramaschi and Velosa, 1996) (Anura:Hylidae) from southern Bahia, Brazil. Herpetology Notes 5:493–494 link
[10] F. C. de Souza-Pinto, I. F. França, C. A. de Mello-Patiu, 2015: Brief description of myiasis cases in three amphibian species from Atlantic Forest located in the central region of the State of Minas Gerais, Brazil. Herpetology Notes, volume 8: 287-290 link
[11] K. C. Pinto, B. C. Padilha, L. S. dos Santos Cruz, G. de Avila Batista, M. D. Pinto Rossi, D. L. Martins, M. P., W. Vaz-Silva, J. M. Neves, 2017: Myiasis caused by Sarcophagidae fly in Dryaderces inframaculata (Boulenger, 1882) (Anura: Hylidae) in the north of Mato Grosso, Brazil. Herpetology Notes, volume 10: 147-149 link
[12] K. Junes, J. Ruiz, E. Quispitupac, 2019: Flesh-fy myiasis (Diptera: Sarcophagidae) in Dendropsophus schubarti (Anura: Hylidae) from Peru. Phyllomedusa: Journal of Herpetology, 18(2), 277-281 link
[13] D. A. Gómez-Hoyos, T. Suárez-Joaqui, O. H. Marín-Gómez, 2012: Flesh fly myiasis (Diptera: Sarcophagidae) in
Pristimantis thectopternus (Anura: Strabomantidae) from Colombia. Herpetology Notes, Volume 5: 27-29 link
[14] C. Kelehear, R. Ibáñez, C. Rodríguez, S. Buitrago, A. A. Durant-Archibold, 2020: Sarcophagid Myiasis in the Bufonid Rhinella alata in Panama. Journal of Wildlife Diseases 56(3), 667-672 link
[15] G. A. Müller, C. R. Lehn, A. Bemvenuti, C. B. Marcondes, 2015: First report of myiasis (Diptera: Sarcophagidae) in anuran of Leptodactylidae (Amphibia). Revista Colombiana de Ciencias Animales. 7. 217-220 link
[16] G. F. Lemos, L. R. Malagoli, R. Lourenço-de-Moraes, Myiasis in three species of Bokermannohyla (Hylidae, Anura). The Herpetological Bulletin 150, 2019: 37-38 link
[17] M. L. Crump, J. A. Pounds, 1985: Lethal parasitism of an aposematic anuran (Atelopus varius) by Notochaeta bufonivora (Diptera:Sarcophagidae). Journal of Parasitology 71:588–591 link
[18] R. Eizemberg, L. T. Sabagh, R. S. Mello, 2008: First record of myiasis in Aplastodiscus arildae (Anura:Hylidae) by Notochaeta bufonivora (Diptera:Sarcophagidae) in the Neotropical area. Parasitology Research 102:329–331 link
[19] F. G. Vázquez-Corzas, A. Sandoval-Comte, P. Hernández-López, S. Ibáñez-Bernal, E. Pineda, 2018: First records of parasitoidism by Sarcophagidae flies (Diptera) on three amphibian species in Mexico. Journal of Natural History 52(35-36):2339-2350 link
[20] C. A. de Mello-Patiu, C. de Luna-Dias, 2010: Myiasis in the Neotropical Amphibian Hypsiboas beckeri (Anura: Hylidae) by a New Species of Lepidodexia (Diptera: Sarcophagidae). Journal of Parasitology 96:685–688 link
[21] P. R. Mulieri, E. F. Schaefer, M. I. Duré, C. E. González, 2018: A new flesh fly species (Diptera: Sarcophagidae) parasitic on leptodactylid frogs. Parasitol Res 117, 809–818 link
[22] M. Hagman, T. Pape, R. Schulte, 2005: Flesh fly myiasis (Diptera, Sarcophagidae) in Peruvian poison frogs genus Epipedobates (Anura, Dendrobatidae). Phyllomedusa 4:69–73 link
[23] C. A. López, T. P. Lavinscky Pereira, M. G. Antúnez, M. E. Peichoto, 2016: Myiasis in the Neotropical amphibian Hypsiboas caingua (Anura: Hylidae) by Megaselia scalaris (Diptera: Phoridae). The Herpetological Bulletin, 38, 2016: 18-20 link
[24] C. A. López, 2019: Miasis por Megaselia scalaris en dos especies de anuros de la Selva Atlántica, provincia de Misiones (Argentina). Boletín de la Asociación Herpetológica Española (2019) 30(2) link
[25] G. R. Mullen, L. A. Durden, 2009: Medical and Veterinary Entomology link
[26] N. Evenhuis, 2006: First record of the frog parasite genus Batrachomyia Krefft from New Guinea (Diptera: Chloropidae). Zootaxa, 1351(1), 53–59. link
[27] R. Dehghani, M. Sedaghat, M. S. Bidgoli, 2012: Wound Myiasis due to Musca domestica (Diptera: Muscidae) in Persian Horned Viper, Pseudocerastes persicus (Squamata: Viperidae). J Arthropod Borne Dis. 2012;6(1):86-9 link
