Salamander fungus / Batrachochytrium salamandrivorans (Bsal)
The skin fungus Batrachochytrium salamandrivorans (Bsal) causes chytridiomycosis, a fatal skin disease, in salamanders and newts. Bsal is related to the other amphibian skin fungus Batrachochytrium dendrobatidis (Bd), which has only been known to science since 1997. Bd and Bsal are estimated to have diverged 50 million years ago (A. Martel et al. 2014 [1]). Bsal causes skin lesions and skin ulcers in caudate amphibians. Often, the 1 to 2 mm skin lesions are surrounded by a sharp margin. In the fire salamander, these black margins are particularly visible (compare Figure 1 & 2); they are somewhat less visible in the upland newt (compare Figure 5). In a later course, larger skin lesions and skin ulcers may occur (compare Figure 3 & 4).

© A. Martel & F. Pasmans (Ghent University)

© A. Martel & F. Pasmans (Ghent University)

© A. Martel & F. Pasmans (Ghent University)

© A. Martel & F. Pasmans (Ghent University)

© A. Martel & F. Pasmans (Ghent University)
Bsal report suspected cases
Bsal suspected cases should be reported immediately. Accumulations of dead animals should always be reported. The earlier the report is made, the better the chances of local action. Contacts for reporting suspected Bsal cases are listed at www.bsaleurope.com/report-cases/ for some countries.
Photos and exact location information are always very useful for scientists. If possible, swabs with cotton swabs or dead specimens in plastic bags can be collected. Specimens should be frozen as soon as possible or, better, cooled and placed in ethanol. If you wait too long, the decomposition process progresses too much and the Bsal fungus can no longer be detected. Try to take as little dirt with you as possible and if possible disinfect your shoes, utensils, etc. Contact the appropriate veterinary office. Testing for wildlife parasites should always be free. Samples can then be dropped off or mailed. Depending on the country concerned, veterinary offices may also send people to collect the samples. An overview of the Bsal diagnostic centers resp. veterinary offices can be found here.
Distribution
Bsal was first described in 2013, after the discovery of massive invasions of fire salamanders in the Netherlands and Belgium. Bsal is native to Southeast Asia and was likely brought to Europe in the pet trade. Newts and salamanders from Southeast Asia can coexist with Bsal without problems. However, in Europe and North America, Bsal can cause great damage. In the Netherlands, the fire salamander is almost completely extinct because of Bsal. Bsal has already been detected in nature in Europe in the following countries: Netherlands, Belgium, Germany (Ruhr, Eifel, Bavaria) and Spain. While the chytrid fungus Bsal became known in the Netherlands, Belgium and Germany mainly due to dead fire salamanders (Salamandra salamandra), it caused mass mortality in a population of marbled newts (Triturus marmoratus) in northern Spain. Frighteningly, the site where it was found in northern Spain is 1000 km from the closest recorded natural outbreak site. Recently (2020), Bsal was also detected in Bavaria, where the skin fungus killed fire salamanders and mountain newts. In Figure 6, the locations where Bsal is found in nature are plotted. Few sites are plotted in the Netherlands and Belgium, although Bsal can be found more widely there than in the other countries.
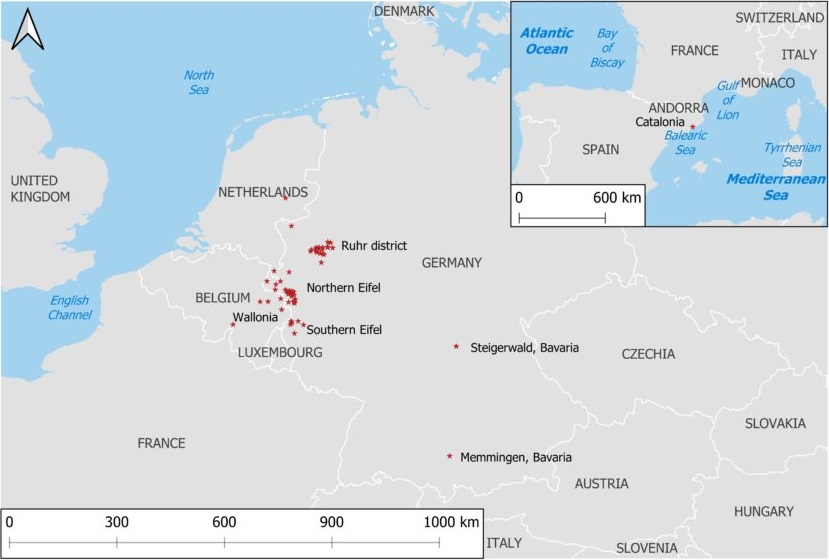
© http://bsaleurope.com/european-distribution/
Species concerned
Most species of newts and salamanders of Europe and North America. Not all species are equally affected. The species that are less affected but still carry the fungus can act as a reservoir and thus spread the fungus. It is also possible that frog amphibians may also act as reservoirs. Some laboratory tests from various studies were published in the “Bsal Action Plan” of the European Commission. The results show that populations of fire salamanders, alpine salamanders and great crested newts are particularly endangered. Less endangered are populations of pond newts and alpine newts. The great crested newt is the least threatened of Bsal and appears to be resistant.
Post-metamorphic amphibians, especially salamanders, are known to be susceptible. However, larval fire salamanders are not susceptible to Bsal (Van Rooi et al. 2015 [2]). Larval susceptibility in other species is still unknown.
Growth conditions
Bsal grows at temperatures between 5°C and 25°C, with the highest growth rate between 10°C and 15°C (Martel et al. 2013 [3]). However, natural infections of Bsal in Asian newts of the genus Tylototriton have been shown to occur at water temperatures as high as 26°C. This more heat tolerant Bsal observation suggests that, as with Bd, there are different variants of Bsal (Laking et al. 2017 [4] & Beukema et al. 2018 [5]).
Treatment
Heat treatment can kill all life stages of Bsal. All Bsal cultures were killed in fire salamanders after 10 days of incubation at 25°C (Compare figure 7 / Bloii et al. 2015 [6]). However, according to recent findings (Schulz et al. 2020 [7]), incubation at 25°C proves effective only after 21 days. If the treatments were too short, the infection persisted and ulceration recurred after 6 weeks.

© Bloii et al. 2015 [6]
There are also Bsal variants heat-tolerant up to 26°C (see the “Growth conditions” section above). Further research on this is unfortunately lacking. It can be assumed that the incubation temperature of 25°C is usually sufficient. Otherwise, a higher temperature can also be selected. The healing success of salamanders and newts can be observed visually.
If the amphibians are heat-sensitive, treatment with antifungals must be carried out. In one study (Bloii et al. 2015 [8]), Bsal infected fire salamanders were cured. Fire salamanders had received a submersion bath in polymyxin E (2000 IU/ml, 10 minutes) twice daily at 20°C for 10 days and were sprayed with voriconazole (12.5 μg/ml) after each bath. This method however did not work at temperatures of 15°C.
Bsal transfer
In the wild, Bsal can be spread through active carriers (e.g. salamanders, newts, anuras) and passive carriers (e.g. birds, water, shoes, equipment). Not all species that are active carriers, i.e. on which Bsal reproduces, die or become ill with Bsal. If Bsal were lethal to all hosts, the chain of infection would be more likely to break off.
Figure 8 shows possible infection routes. In one study (Stegen et al. 2017 [9]), it was shown that the midwife toad and the alpine newt Bsal serve as reservoirs and can transmit the infection to the fire salamander directly or via water (white arrows). Infected fire salamanders can spread the disease when fungal spores spread from their bodies (red arrows). The study authors discovered encapsulated Bsal fungal spores that are more resistant to predation by zooplankton than the motile aquatic form of Bsal zoospores (greater predation indicated by a thicker black arrow). Birds also contribute to the dispersal of Bsal spores as passive carriers. Encapsulated fungal spores floating on the water surface do not exist in Bd. These permanent spores probably enable Bsal to survive for months even in the forest floor.
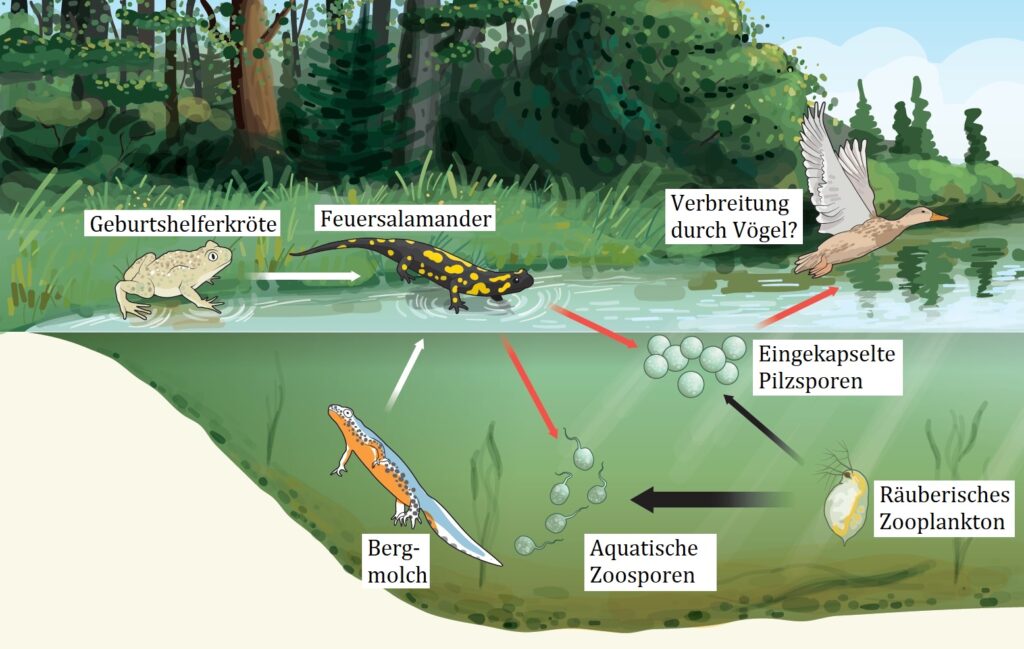
© Matthew Fisher / nature.com
Other hosts: Crayfish, fish, water snails, algae and tadpoles
The chytrid fungus Bd feeds on the keratin of the horny skin parts of the amphibians. For the time being, the tadpoles have no keratinized skin, but only keratinized or keratin-containing mouth parts. Thus, Bd in tadpoles feeds only on the keratin of the keratinized mouthparts and only later on the increasingly keratinized hind legs (McMahon et al. 2014 [10]). Incidentally, keratin or keratin-like substances are also present in the gastrointestinal tract of numerous invertebrates and vertebrates (Schaffeld et al. 2004 [11]). For example, Bd multiplies in the gastrointestinal tract of certain crayfish ((Procambarus alleni, P. clarkii, and Orconectes virilis) and can survive there for at least 12 weeks. Researchers kept 10 uninfected southern leopard frog (Lithobates sphenocephalus) tadpoles in the same container as infected crayfish and found that at the end, the chytrid fungus Bd was found on 7 of 10 of the tadpole mouths (McMahon et al. 2013 [12]). No Bd has been detected on mosquitofish (Gambusia holbrooki) (McMahon et al. 2013 [12]). Likewise, no Bd has been detected on freshwater snails (Physella acuta) or algae of the genus Cladophora (McMahon et al. 2020 [13]).
However, there are different Bd variants that can also recombine with each other and thus rapidly acquire new properties. Therefore, such Bd studies must be viewed with caution and, if possible, repeated with other Bd variants. All of these experiments must also be done with Bsal. It is not yet known whether Bsal can also rely on crayfish or other non-amphibian animals as reservoirs.
Reduced fitness
It can be assumed that a non-lethal Bsal infestation also leads to low fitness and makes the affected amphibians even more susceptible to other parasites. However, in the case of Bsal, there are no scientific studies on this yet. In contrast, in the case of the longer-known Bd, for example, it has been found that Bd-infected tadpoles of common toads (Bufo bufo) survive but show lower fitness (size and jumping power) after metamorphosis (Brilha, 2014 [14]).
Do not enter affected forests
There is currently no treatment method for the salamanders and newts in the wild. In the near and medium future it also seems completely unlikely that possibilities will be found to help on a large scale. The best method is to delay or prevent the spread of the Bsal. It is important to report the occurrence of Bsal, for example sick or dead fire salamanders with recognizable symptoms, in advance. In the Netherlands, it has been forbidden to leave the path in certain affected forests, and posters have been put up for this purpose (compare Figure 9). On the damp shoe, the Bsal fungus can survive longer and be spread to other areas. The posters also recommend to let the shoes dry for a week after walking in the affected area.

© sosvuursalamander.nl
Import / trade ban
European Union, Switzerland and United Kingdom
It is suspected that Bsal was brought to Europe via the pet trade. In order to prevent or limit the further spread of Bsal via animal trade, special provisions have been made for the import and export of all amphibians of the order of caudates (Caudata) to EU countries, Switzerland and the UK; an official veterinary certificate is now required for import. The EU has already issued several implementing decisions on this, the most recent implementing decision for Bsal dated 22 February 2021 can be read here.
Nordamerika (USA, Kanada, Mexiko)
The greatest diversity of salamanders and newts is found in North America, particularly in the United States. Because these species have also shown susceptibility to Bsal in laboratory tests, it is imperative to prevent the importation of Bsal into North America. The U.S. Fish and Wildlife Service therefore imposed a ban on trade in 201 salamander and newt species in January 2016. However, the subsequent discovery that anurans can also transmit Bsal has led to an outcry from scientists calling on the government to ban the import of all amphibian species. Canada imposed import restrictions on all caudate amphibians in May 2017. Mexico, with the world’s second-highest diversity of salamanders, has not yet taken protective measures except for certain risk assessments.
Disinfection
Anyone going on an amphibian tour should disinfect their materials. In a study (Van Rooij et al. 2017 [15]), different disinfectants were tested against Bsal. It is recommended to use 1% Virkon S®, 4% sodium hypochlorite, and 70% ethanol for disinfecting equipment in the field, laboratory, or captivity, with a minimum contact time of 5 minutes for 1% Virkon S® and 1 minute for the latter disinfectants. These conditions are not only efficient against Bsal, but also against Bd and the ranavirus. Hydrogen peroxide, very effective against Bd, is not suitable as a disinfectant for Bsal. PS. If you can’t find your disinfectant, you can disinfect with boiling water.
Is disinfection not possible? Heavy agricultural machinery (compare Figure 10 & 11) should contain as little material as possible before transport. As much muddy soil and vegetation should be removed from the machine as possible.


Bsal detection
Detection by DNA
Duplex real-time PCR method can be used to quantitatively test skin swabs or skin samples for Bsal and Bd simultaneously. It is the best method. DNA sequencing (determination of the letter sequence of the entire genome) of course gives more information also about ancestry and possible Bsal variants. However, DNA sequencing is still far too expensive and time-consuming.
Detection by microscope
There is still no immunochemical detection by microscope that can distinguish Bd from Bsal. However, differentiation by microscopic histology is possible. In Figure 12, the skin from the greenish newt (Notophthalmus viridescens) was compared. Chytridiomycosis from Bd (A) shows mainly chytrid vegetation bodies in superficial keratinocytes. Clearly visible are keratinocytes with intact nuclei (N). In Bsal (B), on the other hand, the chytrid vegetation bodies penetrate deeper into the skin, almost the entire thickness of the keratinocytes is affected.
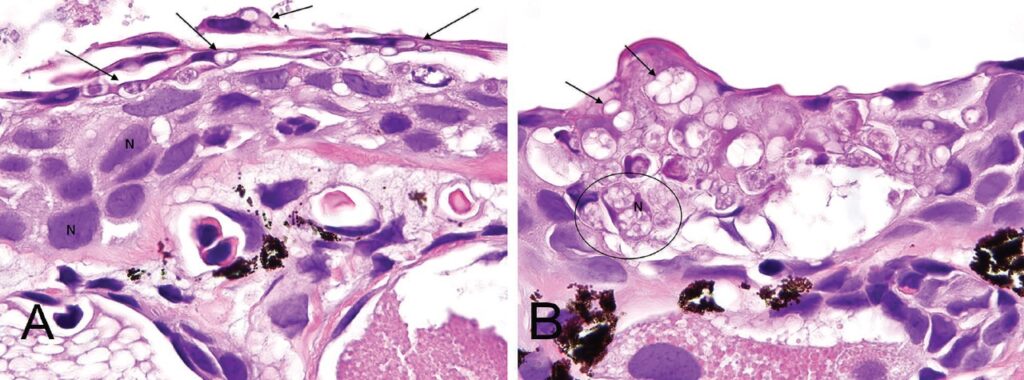
© C. White et al., 2016
Future of Bsal in Europe
According to numerous scientists, Bsal will unfortunately continue to spread. The populations of certain species, especially fire salamanders, will probably collapse in many places, as in the Netherlands. Science is getting better at understanding the processes involved, but methods of destroying Bsal in the wild are unlikely to be discovered. Hypothesis: Combating Bsal in nature requires techniques that are far more complicated and complex than logical inventions such as airplanes, computers, radio signals, etc.
Genetic engineering and ethics
Regardless of whether nature’s amphibians are genetically modified or not, genetic research into why certain species are completely resistant or more resistant to Bsal would be desirable. In the sense of science resp. gene conservation it makes sense to change one gene or a few genes to get millions of genes resp. gene variants. Is it reprehensible to let the fire salamanders die out in nature on a large scale or to make them immune to Bsal with the slightest changes? Crops have been completely bombarded with radioactivity in the past to create new species and traits. In contrast, minor and known changes in DNA sequences would hardly be worth mentioning. What is not ethical is the dragging of numerous creatures around the planet, the whole toxic cocktail of pesticides and drug residues that is unleashed on the environment and the total urbanization and destruction of valuable habitats.
Links
- Bsal Netherlands
- Bsal USA
- Bsal Europe
- www.bsaleurope.com
- Report cases from Europe
- www.bsaleurope.com/report-cases/ (including Switzerland)
- Diagnostic centers for Bsal tests in Europe
- Instructional videos
- Report cases from Europe
- www.bsaleurope.com
- www.niklas-wildlife.com/batrachochyrium-salamadrivorans
- Bsal Projects
- Bochum
- University of Trier
- University of Gießen
- University of Leipzig
- Fundmeldesystem der Biologischen Station Mittlere Wupper (NRW)
- Bsal Projects
List of sources
[1] A. Martel, M. Blooi, C. Adriaensen, P. Van Rooi, W. Beukema, M. C. Fisher, R. A. Farrer, B. R. Schmidt, U. Tobler, K. Goka, K. R. Lips, C. Muletz, K. R. Zamudio, J. Bosch, S. Lötters, E. Wombwell, T. W. J. Garner, A. A. Cunningham, A. Spitzen-van der Sluijs, S. Salvidio, R. Ducatelle, K. Nishikawa, T. T. Nguyen, J. E. Kolby, I. Van Bocxlaer, F. Bossuyt, F. Pasmans, 2014: Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science 346:630–631 link
[2] P. Van Rooi , A. Martel, F. Haesebrouck, F. Pasmans, 2015: Amphibian chytridiomycosis: a review with focus on fungus-host interactions. Veterinary Research 46, Article number: 137 link
[3] A. Martel, A. Spitzen-van der Sluijs, M. Blooi, W. Bert, R. Ducatelle, M. C. Fisher,
A. Woeltjes, W. Bosman, K. Chiers, F. Bossuyt, F. Pasmans, 2013: Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. PNAS September 17, 2013 110 (38) 15325-15329 link
[4] A. E. Laking, H. N. Ngo, F. Pasmans, A. Martel, T. T. Nguyen, 2017: Batrachochytrium salamandrivorans is the predominant chytrid fungus in Vietnamese salamanders. Scientific Reports 7, 44443 (2017) link
[5] W. Beukema, A. Martel, T. T. Nguyen, K. Goka, D. S. Schmeller, Z. Yuan, A. E. Laking, T. Q. Nguyen, C. Lin, J. Shelton, A. Loyau, F. Pasmans, 2018: Environmental context and differences between native and invasive observed niches of Batrachochytrium salamandrivorans affect invasion risk assessments in the Western Palaearctic. Diversity and Distributions, Volume 24, Issue 12, 1788-1801 link
[6] M. Blooi, A. Marte, F. Haesebrouck, F. Vercammen, D. Bonte, F. Pasmans, 2015: Treatment of urodelans based on temperature dependent infection dynamics of Batrachochytrium salamandrivorans. Scientific Reports 5, 8037 link
[7] V. Schulz, A. Schulz, M. Klamke, K. Preissler, J. Sabino-Pinto, M. Müsken, M. Schlüpmann, L. Heldt, F. Kamprad, J. Enss, M. Schweinsberg, J. Virgo, H. Rau, M. Veith, S. Lötters, N. Wagner, S. Steinfartz, M. Vences, 2020: Batrachochytrium salamandrivorans in the ruhr district, germany: History, distribution, decline dynamics and disease symptoms of the salamander plague. SALAMANDRA 56(3): 189–214 link
[8] M. Blooi, F. Pasmans, L. Rouffaer, F. Haesebrouck, F. Vercammen, A. Martel, 2015: Successful treatment of Batrachochytrium salamandrivorans infections in salamanders requires synergy between voriconazole, polymyxin E and temperature. Scientific Reports 5, 11788 (2015) link
[9] G. Stegen, F. Pasmans, B. R. Schmidt, L. O. Rouffaer, S. Van Praet, M. Schaub, S. Canessa, A. Laudelout, T. Kinet, C. Adriaensen, F. Haesebrouck, W. Bert, F. Bossuyt, A. Martel, 2017: Drivers of salamander extirpation mediated by Batrachochytrium salamandrivorans. Nature 544: 353-356 link
[10] T. A. McMahon, J. R. Rohr, 2014: Transition of chytrid fungus infection from mouthparts to hind limbs during amphibian metamorphosis. Ecohealth. EcoHealth, Volume 12, 188–193 link
[11] M. Schaffeld, J. Markl, 2004: Fish keratins. Methods Cell Biol, 78:627–671 link
[12] T. A. McMahon , L. A. Brannelly, M. Chatfield, P. Johnson, M. Joseph, V. J. McKenzie, C. Zawacki, M. D. Venesky, J. R. Rohr, 2013: Chytrid fungus Batrachochytrium dendrobatidis has nonamphibian hosts and releases chemicals that cause pathology in the absence of infection. PNAS January 2, 2013 110 (1) 210-215 link
[13] T. A. McMahon, C. Nordheim, D. Propokiak, 2020: Freshwater snails and the green algae Cladophora are probably not hosts of Batrachochytrium dendrobatidis. Freshwater Biology, 66 (3) link
[14] P. M. Brilha Patrício, 2014: How does exposure to the fungus Batrachochytrium dendrobatidis affects the tadpoles of the common toad, Bufo bufo, under different stresses? Dissertação de Mestrado em Biologia da Conservação, Universidade de Lisboa link
[15] P. Van Rooij, F. Pasmans, Y. Coen, A. Martel, 2017: Efficacy of chemical disinfectants for the containment of the salamander chytrid fungus Batrachochytrium salamandrivorans. PLoS One. 2017 Oct 12;12(10):e0186269 link
